Summer Research Program for Science Teachers/Partners in Science
Mary-anne Garcia
Malverne H.S.
2002
Covalent Bond
Aim: How do we describe Covalent Bond?
I.O./SWABT 1. Define covalent bond.
2. Distinguish between a molecular formula and a structural formula.
3. Explain the difference between single, double, and triple covalent bonds.
New York State Learning Standards:
The following New York Standards are addressed in this lesson.
Students will understand and apply scientific concepts, principles, and theories pertaining to the physical setting and living environment and recognize the historical development of ideas in science.
Students will apply technological knowledge and skills to design, construct, use, and evaluate products and systems to satisfy human and environmental needs.
Standard 6: Interconnectedness: Common Themes
Students will understand the relationships and common themes that connect mathematics, science, and technology and apply the themes to these and other areas of learning.
Standard 7: Interdisciplinary Problem Solving
Students will apply the knowledge and thinking skills of mathematics, science, and technology to address real-life problems and make informed decisions.
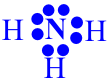
Motivation: Ask the students to examine the Lewis Dot structure for ammonia.

PQ Ammonia solutions are used to clean, bleach, and deodorize; to etch aluminum; to saponify (hydolyze) oils and fats; and in chemical manufacture. The ammonia sold for household use is a dilute water solution of ammonia in which ammonium hydroxide is the active cleansing agent. Is the octet rule satisfied for each atom in the ammonia molecule?
*Octet rule is the tendency for elements to gain, lose, or share electrons so that the
valence shell is filled with eight electrons is known as the octet rule.
PQ Why is the octet rule satisfied for hydrogen with only two valence electrons? The
first energy level is full with 2 electrons.
PQ Paper can be easily colored, cut, and folded. These properties are put to attractive use
in the Japanese art of origami. What kind of bonds unites the atoms in the molecules of a
sheet of paper?

1. are formed by sharing pair of electrons between two atoms. They are found in a long list of substances such as paper, garbage bags, sugar, bloodstream, DNA, and every tissue in the human body.
2. Molecular substance is a substance that has atoms held together by covalent bonds
3. A molecule may contain as many as few as 2 atoms or as many as thousand or even a million atoms. Examples:
Oxygen, a gas that travels through your bloodstream,
Hydrogen is a colorless, odorless gas that accounts for 75 percent of the universe's mass. It's also used in NASA's space program as fuel for the space shuttles, and in fuel cells that provide heat, electricity and drinking water for astronauts.
Nitrogen gas makes up about 78% of the atmosphere by volume
Commercially, CO2 finds uses as a refrigerant (dry ice is solid CO2), in beverage carbonation, and in fire extinguishers.
E. Larger molecules such as DNA and polyethylene
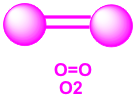
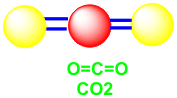
· Describe single, double and triple bonds.
4. Types of bonds based on number of pairs of shared electrons.
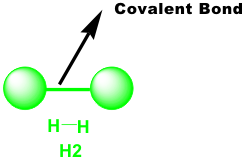
a. Single covalent bond – 2 atoms share exactly one pair of electrons. Examples: H2, F2, I2 and other diatomic molecules are molecules that contain single covalent bonds.
b. Double covalent bond- consists of two pairs of shared electrons. Examples: O2, CO2 and H2CO (formaldehyde) are molecules that contain double bond.
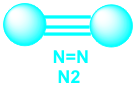
c. Triple covalent bonds- consist of three pairs of shared electrons. Examples: C2H2 (ethyne) and N2 are molecules that contain triple bonds.
5. Molecular formula describes the composition of a molecular compound. It tells you how many atoms are in a single molecule of the compound. Example, the molecular formula for molecular nitrogen is N2, which consists of two oxygen atoms.
PQ Ask the students for the molecular formula of sucrose or ordinary table sugar. C12H22O11. This molecular formula indicates that this molecule contains 12 C atoms, 22 hydrogen atoms, and 11 oxygen atoms.
6. Structural formula specifies which atoms are bonded to each other in a molecule. One type of structural formula is the Lewis structure for molecules that is based on the Lewis dot diagrams for atoms.
PQ What information could be derived from the Lewis structure below?
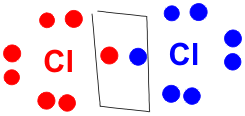
· Explain to the class that the diagram is the Lewis dot formula for molecular chlorine (Cl2). The boxed dots signify the electrons in a single bond. The dots placed around the element symbol represent the atom’s valence electrons. The dots placed between the two element symbols represent the electrons in a covalent bond. The Lewis structure above also tells you that the molecule contains 2 chlorine atoms joined but a single covalent bond.
Challenge students to draw the Lewis structures of molecules containing single, double and triple bonds such as H2, O2, N2, CO2, H2CO and C2H2.
Summary: Have students present orally of any concepts on covalent bonding they learned for the day.
Reinforcement Lesson Using the Plato Educational Software: Have students explore the lesson on covalent bonding via Internet using the Website, Cyberedonline.com. Challenge students to answer the pretest questions.