The design of this lab is mainly from Dr. Joan Boorman of the State University of New York at Albany, member of the Physics Teacher Institute. I adapted the lab to meet the needs and level of my students. The format, however, is inspired by Lowel Sylvester, physics teacher at East Aurora and physics mentor.
NAME:___________________
LAB GROUP: A B C D E
CLASS PERIOD #:
PARTNERS:______________
______________
______________
DATE DUE:____________________
DATE REC:_______ GRADE_____
LATE POINTS_____
FINAL SCORE_____
CHECK LIST FOR LAB:
DO YOU HAVE:
An abstract including:
In this lab you will observe the spectra of hydrogen and mercury
(as gases under low pressure) from which you will gain an
understanding of how spectra can be used to identify substances
anywhere in the Universe. You will also have to determine the
energy associated with photon emission. [Content Standard B- Structure of atoms] [Content Standard B- Matter/Energy Interactions]
When a substance absorbs energy (from heat or high voltage applied to a gas discharge tube, or photon absorption, etc.) electrons are raised to higher energy state. When they drop back to their original levels, the absorbed energy is released as photon emission (visible or invisible light). The frequency, (thus the color) of this light is directly proportional to the energy difference between the initial and the final energy levels. In 1885, J. J. Balmer found an empirical formula that correctly gives the wavelengths of hydrogen emission in the visible range of the Electromagnetic Spectrum.
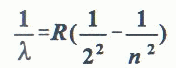
Since the structure of atoms differ from element to element, the possible transition between energy levels within atoms of each element is also distinctive. Thus, each element is capable of displaying a characteristic array of colors, or spectrum. Gases under low pressure emit characteristic bright-line spectra, which are like finger-prints for that element. [Content Standard Unifying Concepts- Systems, order, and organization]
The energy emitted can be calculated by: E=h.f
Spectroscope
high voltage source
hydrogen and mercury discharge tubes
color |
|
|
f in Hz |
energy (J) |