Corrosion/Plating/Electrolysis
Jonathan M. Peter
New Explorations into Science Technology and Math, Manhattan
Summer Research Program for Science Teachers
Summer 2006
Rationale:
The following lab is conducted after students have had a lecture(s) and homework problems pertaining to the specific content area that is covered in the lab. For example, students will be familiar with half cell reactions, potential, circuits, etc. The lab itself, however, will be new material that builds on previous classroom content. Ideally, this is an AP physics lab. During the lab students will follow a worksheet that guides them through an inquiry pertaining to the content. Graduate students, the high school instructor and/or the university professor will assist students in their experiment.
After the experiment students will complete a series of questions related to and extending the lab experience and will write up the lab in a formal report. This lab requires Lab View software, data collection program, deionized water, NaCl, solid piece of iron, a penny, a quarter, a piece of copper, possibly perchloric acid.
Background information
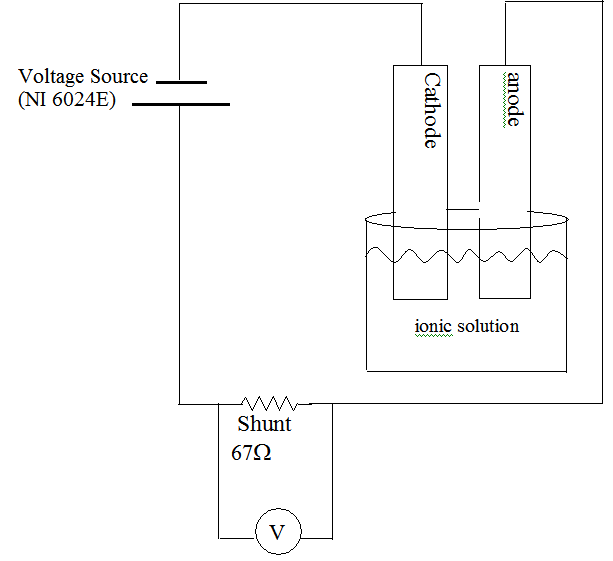
During this demonstration, two metals are placed in an ionic solution. A voltage is applied across these two terminals and the current is measured. At low voltages, little or no current is measured. At larger voltages, a significant current is measured. Delineating these two regimes is a characteristic voltage where a small increase in voltage produces a large current change. Using data acquisition and real time plotting, students can watch the voltage sweep (increase) and observe the sudden increase in current at the characteristic voltage. At the conclusion of this demonstration, the anode has been corroded and the cathode has been plated.
Do Now:
Take out your notebooks, work quietly for 2 minutes and describe what you know about corrosion. Specifically, what things corrode and under what conditions do they corrode?
Teacher: Collect (verbally) the student suggestions and briefly highlight any re-currying themes, jot them on the board. Water, iron and rust should come up as well as possibly copper. Teacher highlights and reviews the following items
- iron à rust oxidation reaction.
- In oxidation reactions, there is a loss of electrons (L.E.O says G.E.R.)
- loss of electrons results in a positive charge
- charging is a transient property. Charged objects will attract / give up electrons from an available source and will become neutral.
- Current is moving electrons
Teacher asks what would happen if one supplied iron with an excess of electrons. [Iron naturally will give up electrons in ionic solutions, if you supply extra electrons to the iron, it will give them up more readily.] Students should realize that the current is analogous to the current flow. Current can be measured. Teacher asks: if we wish to speed the corrosion, should we connect the iron to the positive or the negative of the battery. Students should realize that the iron requires electrons to be added, and therefore need to be connected to the negative terminal. This is the anode and this is where oxidation happens. The other positive terminal is connected to cathode where reduction happens. Teacher draws the following diagram (bare) and then has students label the terminals, etc.
Students observe the projected computer screen with lab view software. Teacher describes the anode and cathode and computer screen. Put two pieces of iron into ionic solution. Run the data collection. Data is graphed. Use a digital projector to expand and discuss the data findings with the students.
- When does ionization happen?
- Are there any interesting things that the data show?
Run the voltage for a while (10 minutes). Remove the samples from the solution. Visually inspect the samples and ask students for hypothesis and theory for their disparate appearances. The anode has been oxidized (rusted) the cathode has not.
Rinse samples in and replace solution with deionized water. Run data collection again. Ask students what they notice now. Why does the reaction happen at a higher voltage?

Possible ideas: Take a penny and a quarter, make quarter cathode, penny, anode. Quarter turns copper color, penny corrodes. Then reverse operation to remove copper color from quarter.
These metals could be replaced by two spontaneously reacting metals that would otherwise produce a galvanic cell. Students could then see the output voltage and could increase the voltage across the terminals until the current ceased. Finally, an experiment can be designed where students find the optimum ionic concentration for the quickest reaction.