Nano: What Our Eyes Can't See
Isaac E. Young Middle School, Westchester
Summer Research Program for Science Teachers
Summer 2007
Time: 1-2 Hours
Level: 6th Grade
Materials:
SEM images
nanotubes
metric prefixes chart
large piece of paper
thin tip marker
strip of paper 216 mm x 5 mm
microscopy graph
Objectives:
1) Students will understand the concept of nano and its relation to other scales in the metric system.
2) Students will work cooperatively with partner and cut paper in half as many times as possible to understand concept of nano.
3)
Students will analyze science graph, charts, and
images dealing with the nano scale.
Motivation:
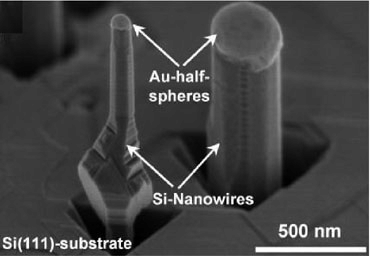
Place an SEM picture of silicon nanowires with gold coating and ask the students “How large are these nanowires? Show students where the scale is and ask them “What does nm stands for?” (Fig 1.)

Fig 1. Nanowires with gold spheres
Development of Lesson:
After listening to their responses, handout a list of metric prefixes (Fig. 2) to the students and review each prefix with them. Next, explain that “nano” is a prefix for one billionth. A nanometer (nm) is a billionth of a meter.
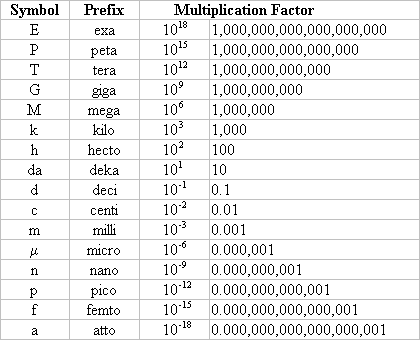
Fig 2.
Metric Prefixes
Next, Take a large piece of paper and draw a 1-meter x 1-meter box on both sides of the paper. Explain that a nanometer is approximately the size of 1/100,000 the thickness of a human hair, or one-billionth the size of the box. Have a student draw a millimeter box in the meter box. Have a student stand inside the box and tell the class “if the student inside the box is the size of a hair then that box would be the size of a human blood cell.1 Now turn the paper over to reveal the other box. Explain to the students that if the student standing in the box were a blood cell then the millimeter box would be around the size of a nanometer.
Next, Explain to the students what nanotubes are and their potential uses.2 Show them pictures from SEM of nanotubes (Fig 3.) and actual nanotubes in a centrifuge tube. Ask the students “Why do you think they are called nanotubes?” Have students use the scale to estimate their size.
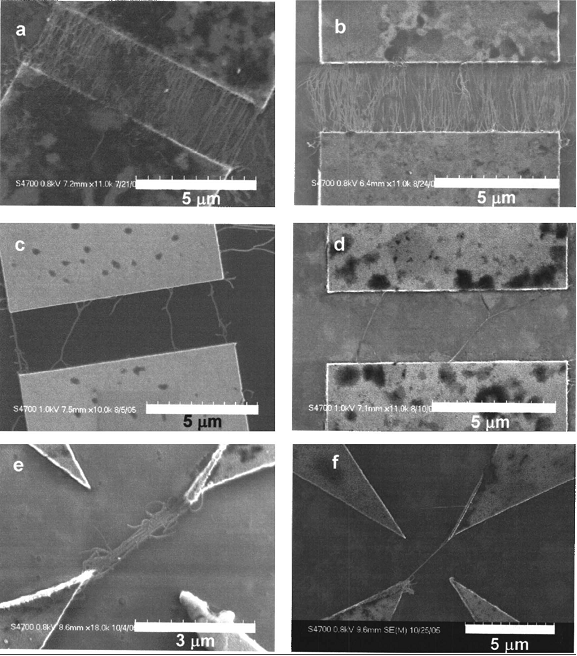
Fig 3. Nanotubes suspended across electrodes
Next, Hand out a strip of paper, 216 mm x 5 mm, to each pair of students. Ask the students “How many times will you need to cut the paper to get a 10 nanometer long piece of paper?” Have the students answer the following questions when they are done cutting the paper.
1. How many times were you able to cut the paper?
2. How close was your smallest piece to the nanoscale?
3. Why did you have to stop cutting?
4. Can macroscale objects, like scissors, be used on the nanoscale?
5. Can you think of any way to cut the paper any smaller?
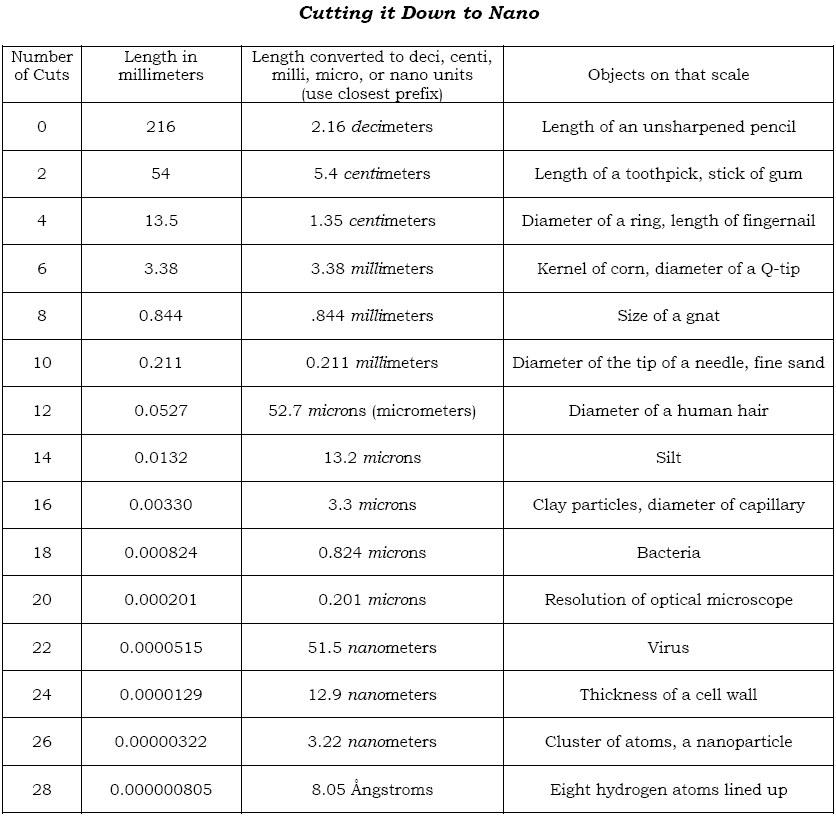
Fig 4.
Scale Chart
Summary:
Go to the following website “The Universe Within” and watch as the tutorial moves from 10,000,000 light years out towards earth in successive orders of magnitude all the way down to a quark in an atom.
Assessment:
Hand out a graph on microscopy (Fig 6.), and have the
students answer the following questions.4
1)
What is
a microscope?
2)
When
was the first optical microscope used?
3)
Can we
see nanotubes with an optical microscope?
Why or why not?
4)
Around
what year were we able to see DNA?
5)
Why is
the naked eye line continually straight?
6)
Why
does the optical microscopy line move down as time moves on?
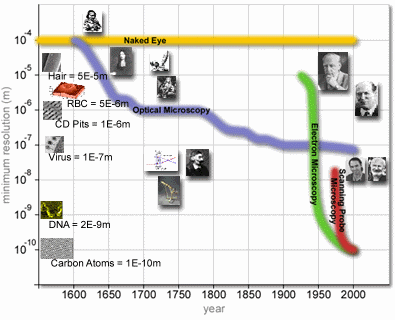
Fig 6.
Microscopy/Time Graph
1. Standard 3. S3.1b generate and use
scales, create legends, and appropriately label axes. The grouping of magnitudes
of size, time, frequency, and pressures or other units of measurement into a
series of relative order provides a useful way to deal with the immense range
and the changes in scale that affect the behavior and design of systems.
2. Standard
3.3c Atoms may join together in well-defined molecules or may
be arranged in regular geometric patterns.
3. Standard 3.2 Use powers of ten notation
to represent very small and very large numbers.
4. Standard 3. S3.1b generate and use
scales, create legends, and appropriately label axes