Melting Ice with Road Salt: An Inquiry-based Project
Rachel
Matundan
PS/IS 119
The Glendale School, Queens
Summer
Research Program for Science Teachers
August
2012
Subject: General Science, Chemistry
& the Scientific Method
Grade Level:
6-8
Unit:
Chemical Interactions Unit
Time required:
Multiple 43 minute class periods (pace depends on students)
Activity Overview:
In this extended lesson, students are challenged to formulate a question, design
and perform an experiment, and compose a final product based on an initial
observation about salt on ice. This is intended to be an open-ended inquiry
lesson using the 5E model as a guide. The left column includes various
strategies and the check (Ö)
indicates which strategy is used in that section of the 5E. Some inquiry
questions and rubric was modified from the provided reference.
Reference:
Wheeler, L. & Bell, R. Open-Ended Inquiry.
The Science Teacher, 2012. 79(6): 32-39.
Objectives:
1.
Scientific
Inquiry- The central purpose of scientific inquiry is to develop explanations of
natural phenomena in a continuing, creative process. Engineering Design-
Engineering design is an iterative process involving modeling and optimization.
(NYC Intermediate Core Curriculum Grades 5-8; Standard 1 “Analysis, Inquiry &
Design”)
2.
The rate of solution can be affected by the size of the
particles, stirring, temperature, and the amount of solute already dissolved
(3.1b). The phase in which matter exists depends on the attractive forces among
its particles (3.1c). Examples of physical changes include freezing, melting,
condensation, boiling, evaporation, tearing and crushing (3.2a). (Physical
Settings: 3)
3.
Follow precisely a multistep procedure when carrying out
experiments, taking measurements, or performing technical tasks (Reading #3).
Introduce a claim (Writing #1a) & support claim with logical reasoning and
relevant, accurate data and evidence (Writing #1b). (Common Core State Standards
in Science)
Key Vocabulary:
Phases of matter, atom, molecule,
melting, independent variable, dependent variable, constants
Materials:
See the materials and handouts within each day / lesson.
Procedures: See the procedure
within each day / lesson.
|
(Day
1) Challenge: What occurs when salt and ice mix?
|
|
Do Now:
Preassessment, prior knowledge & engaging the learners.
Draw how
atoms appear at the different phases of matter.
|
Quiz, test
Surveys
KWL
Journals
Reflection
ÖBrainstorm
Concept
formation
Thumb it
|
|
(Engage/Explain)
Watch 19
second video “Impact
of Ice on Melting Ice Cube”
|
Lecturette
Presentation
Demonstration
ÖVideo
Field trip
Guest
speaker
Text
|
|
(Explore) Tasks:
Grouping- heterogeneous based on
strengths/ weaknesses in ELA and Math state scores.
Experimental
Design Part 1: Based on the video clip respond to the following
questions…
1.
What questions come to mind after watching this video?
2.
What materials are readily available for conducting this experiment?
(Each group will have a tray of containers, sodium chloride, calcium
chloride, graduated cylinder, spoons, timer, post it with the words
“crushed ice” and “ice cubes”).
3.
How does the salt on ice act?
4.
How can I change the materials to affect the salt on ice?
5.
How can I measure and describe the response of salt on ice to the change
in #3?
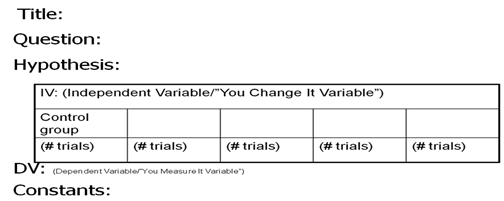
Complete
Experimental Design Diagram:
|
Jigsaw
Learning
centers
Projects
Compact/
enrichment
ÖConcept
maps, graphic organizers
ÖProblem
based inquiry
Research
Independent
study
Model
Role play
Reciprocal
teaching
Peer
reading/ editing
|
|
(Extend)
Homework: Based on today’s lesson, research on why the addition of salt
might affect the melting of ice. Cite your sources.
|
|
|
(Evaluate):
Assessment: Check “Experimental Design Diagram”.
Discuss your
group’s responses to the questions and as a group, decide on one
question they would like to answer by doing an experiment.
|
Exit slip
Quiz, test
Performance
Products
Presentation
Demonstration
Log, journal
Checklist
Portfolio
Rubric
ÖShare
out
|
|
(Day
2) Challenge: What occurs when salt and ice mix?
|
|
Do Now:
Preassessment, prior knowledge & engaging the learners.
Think-Pair-Share last night’s homework assignment. What molecules are
involved in the experiment? How do you think the molecules are behaving
and interacting?
|
Quiz, test
Surveys
KWL
Journals
ÖReflection
Brainstorm
Concept
formation
Thumb it
|
|
(Engage/
Explain)
|
Lecturette
Presentation
Demonstration
Video
Field trip
Guest
speaker
Text
|
|
(Explore) Tasks
Experimental
Design Part 2: Write a procedure and data table to record data. Decide
individual duties before, during and after the experiment. Once approved
by teacher, begin experiment.
|
Jigsaw
Learning
centers
Projects
Compact/
enrichment
Concept
maps, graphic organizers
ÖProblem
based inquiry
Research
Independent
study
Model
ÖRole
play
Reciprocal
teaching
Peer
reading/ editing
|
|
(Extend)
Homework: What challenges did your group face during today’s lesson?
|
|
|
(Evaluate)
Assessment
During
class, meet with students who performed low on the ELA (regarding
procedure) and Math (regarding data table) state exams.
|
Exit slip
Quiz, test
ÖPerformance
Products
Presentation
Demonstration
Log, journal
Checklist
Portfolio
Rubric
Share out
|
|
(Days 3-6) Challenge: What occurs when salt and ice mix?
|
|
Do Now:
Preassessment, prior knowledge & engaging the learners.
Think-Pair-Share last night’s homework assignment. Students are asked to
respond with possible solutions to troubleshooting issues.
|
Quiz, test
Surveys
KWL
Journals
ÖReflection
Brainstorm
Concept
formation
Thumb it
|
|
(Engage/
Explain)
|
Lecturette
Presentation
Demonstration
Video
Field trip
Guest
speaker
Text
|
|
(Explore) Tasks
Complete
experiment and analyze data. Begin brainstorming on final product.
Discuss individual duties for final product (see rubric below).
|
Jigsaw
Learning
centers
Projects
Compact/
enrichment
Concept
maps, graphic organizers
ÖProblem
based inquiry
Research
Independent
study
Model
ÖRole
play
ÖReciprocal
teaching
Peer
reading/ editing
|
|
(Extend)
Homework: Work on final product.
|
|
|
(Evaluate)
Assessment- See rubric below.
|
Exit slip
Quiz, test
ÖPerformance
ÖProducts
ÖPresentation
Demonstration
Log, journal
Checklist
Portfolio
ÖRubric
Share out
|
|
Rubric for
Students’ Final Product
|
|
Type of
Product (check one)
|
___Poster
|
___PowerPoint
|
___Lab
Report
|
___Other
|
|
*All
products MUST include the
following…
|
|
|
Full Credit
|
Partial Credit
|
No Credit
|
|
|
Experimental
Details
|
Includes:
-
independent variable
-
dependent variable
-
constants
-
hypothesis
|
One or two
of the details are missing.
|
Experimental
details are not addressed in the report.
|
|
|
Data
|
The data are
presented in a clear, concise, manner (data table, graph, or other
visual representation) and properly labeled.
|
Labels are
missing, the data is unclear or the best representation for the data is
not used.
|
The results
are missing from the report or not legible.
|
|
|
Results
|
A conclusion
paragraph about the relationship of the data (claim) is written and
based upon evidence from the experiment. The hypothesis is also
addressed.
|
A conclusion
is made, but no evidence is used to support the conclusion or the
conclusion does not address the hypothesis.
|
The
conclusion contradicts the data or is absent from the report.
|
|
|
Explanation
(reasoning)
|
A viable
process underlying the observed phenomenon is explained (written or
graphical representations with labels are acceptable) and supported by
evidence from the data and research sources.
|
The
explanation is incomplete. It does not address the main relationship
described in the results or does not use data to support the
explanation.
|
The
explanation contradicts the observations or is absent from the report.
|
|
|
Evaluation
|
The overall
inquiry activity is evaluated through the following questions:
-
How reliable are the data?
(number of trials, human error)
-
How might you change the
experiment in the future?
-
What further scientific
questions arise from this activity? How might you go about finding
answers to these questions?
|
One of the
three questions is not addressed or answers to each question are
incomplete.
|
One sentence
or less is given for each question or the evaluation is absent from
product.
|
|
