



Huntington H.S., Long Island
Summer Research Program for Science Teachers
August 2011
Subject: Environmental Science
Grade Levels: 9-12
Time Requirements: Approximately 15 minutes preparation and 2 hours class time
Topics Covered by This Lesson: Cloud formation and air pollution
Teaching Objectives:
Teacher will introduce the topic of air pollution and provide accompanying note sheets and handouts for lab activity
Student Objectives:
Students will be able to define air pollution and use basic terminologies used
in describing air pollution i.e. (haze, aerosols, air quality, ozone, smog) and
determine why air quality is important to living things.
Ties to Science Standards:
Students will perform a scientific experiment using appropriate methods and with careful observations.
Students will communicate the results of their experiment in a well defined statement.
Introduction:
The troposphere is the lowest level of the atmosphere.
It extends approximately 10-15 kilometers vertically into the air. To put this in perspective, the Empire States Building in New York City
is only 381 meters tall. When
we talk about air pollution we are always
referring to this region.
What is Air Pollution?
Air pollution is a condition where the concentrations of various substances due to anthropogenic factors (human caused) are present in higher concentrations than what is considered normal.
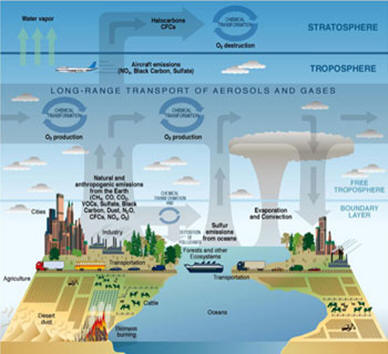
Figure 1 Source: Courtesy United States Climate Change
Science Program (Illustrated by P. Rekacewicz)
Aerosols are very, very fine solid or liquid particles suspended in a gas. These particles range in size from a few nanometers to only 10’s of micrometers large. This is really a small numerical value; a human hair is 100um thick. Some of the aerosols found in the troposphere are the result of natural sources produced from the decomposition of living things (plants and animals), volcanic eruptions, and ocean movement and sea spray. These aerosols in general do not have long lives in the atmosphere. They typically will survive in the atmosphere for time periods from a few days to one week. However, even with this short lifespan the presence of aerosols can alter the chemistry of clouds. However, the large amounts of Aerosols found in the troposphere are largely anthropogenic in nature. They are the result of emissions from human factors such as the burning of biodiesel fuels or in the production of manufactured products (see Figure 1).
The
formation of clouds is part of the water cycle.
The sun through the process of evaporation or transpiration provides
water into the atmosphere forming clouds. A cloud is a collection of extremely
small water droplets or ice crystals.
These droplets are so tiny they can float in the air.
In order for a cloud to form several processes must converge.
The water from either a land mass or sea mass must evaporate and collect
on aerosols or other dust particulate matter in the air.
This process is called seeding.
Once enough of the water droplets have been seeded a visible cloud is
formed. See Figure 2 below for various scenarios for cloud formation.
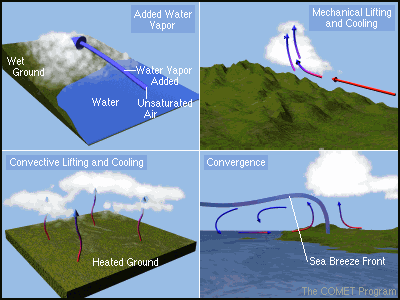
Figure 2. Picture captured from: http://earthsci.org/processes/weather/weaimages/cldform.gif
Most clouds are white because they reflect the light from the sun. Note**Storm clouds appear darker because they are thicker than other clouds so less light is transmitted through. However, in areas susceptible to high levels of air pollution called hotspots you may notice that the clouds present themselves in various shades of red, orange or brown; theses colors when present are commonly called smog. The term smog originally referred to a mix of smoke and fog. However, today we have expanded its definition to include any type of aerosol contamination in the air. Where there are high levels of air pollution there will be greater cloud cover. Cloud cover is the obscuring of light due to the presence of clouds. The chemical makeup of these clouds can impact the environment in not so obvious ways. For example, pollution composed of various organic materials can cause cloud formation that are thinner than unpolluted clouds which can increase the amount of harmful radiation that reaches the earth’s surface. Or the clouds can be denser and trap harmful ozone and other materials in the troposphere.
At this point it is necessary to distinguish between
photochemical smog which the air
pollution that creates the haze that you see surrounding an urban city or
metropolis from air pollution that is found in cloud formation.
The two are not exclusive of each other but the effects are notably
different. In another lesson we
will discuss photochemical smog. In
Activity 1, we will study the affect of different types of particulate
matter on the formation of clouds.
National Science Standards