Summer Research Program for Science Teachers/Partners in Science
Horace E. Walcott
Brooklyn Technical High School
2002
The Flame and the Atom:
Rutherford’s Gold Foil Experiment
Aim: How is the atom structured?
National Standards
Content Standard A:
As a result of activities in grades 9-12, all students should develop
· Abilities necessary to do scientific inquiry
· Understanding about scientific inquiry
Content Standard B:
As a result of their activities in grades 9-12, all students should develop an understanding of
· Structure of atoms
· Structure and properties of matter
· Chemical reactions
· Motion and Forces
· Conservation of energy and increase in disorder
· Interaction of energy and matter
Content Standard E:
As a result of activities in grades 9-12, all students should develop
· Abilities of technological design
· Understanding about science and technology
Content Standard F:
As a result of activities in grades 9-12, all students should develop understanding of
· Personal and community health
· Population growth
· Natural resources
· Environmental quality
· Natural and human induced hazards
· Science and technology in local, national, and global challenges
Content Standard G:
As a result of activities in grades 9-12, all students should develop understanding of
· Science as a human endeavor
· Nature of scientific knowledge
· Historical perspectives
This laboratory exercise will be one of investigations of the mini-unit on The Flame and The Atom. It will require two 45 minute periods.
I. Understand the science and technology of the instruments used in the deciphering the atom
II. Hone their skills in scientific investigation
III. Gain insight into the personal and social perspectives of science
IV. Compare the Rutherford Model of the atom with the Bohr Model and the Quantum Mechanical Model of the atom
V. Apply scientific reasoning to problem-solving in chemistry
Why is atomic security more critical in the 21 st century than it was in the 20th century?
They will observe a motion movie, in ms-Power Point of alpha particles interacting with a gold foil in a cloud chamber. [Figures 1 B through D]
The path of the alpha particles were previously recorded by an Intel microscope positioned over a cloud chamber in lead glass radiation protection case. [Figure 1 A]
Each team member will record his or her observations and an explanation of the observations. The principal investigator of each team will then ensure that each team member has a functioning geometric kit. Each team member will use protractors and compasses to measure the path angles of the alpha particles in two of the frames. [Figures 2A and 2B]
The data analyst for each group will record the path angles on a table on the board. The principal investigator of each team will be assisted by the team’s data analyst in preparing a radial graph of the path angles of the alpha particles. The variables will be path angle and number of alpha particles with a specific path angle.
Medial Summary
Why are the alpha particles with path angles of 180 ° significant?
Why are the alpha particles with path angles greater than or less than 180 ° significant?
The principal investigator and the data analyst of each team will facilitate a discussion of the data sets. Each team will draw a structure of the atom using the analyzed data and the radial graphs.
Final Summary
Why are the radial graphs similar to the photographs of the impacted particles? How can you use the path angles to construct a model of the atom?
Read
Study three internet websites on atomic structure, which discuss the models of the atom. Be prepared to evaluate the scientific validity of the Rutherford model, the Bohr model and the Quantum Mechanical model.
Portfolio Assignment
Why was the Cathode Ray Tube (CRT) a significant instrument in deciphering the structure of matter? Contact a scientist on the internet and interview researcher on the role of CRT or related technology in instruments used in his or her laboratory.
[Due after 3 weeks]

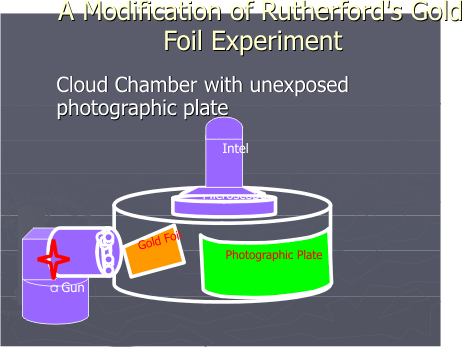
Figure 1A. Rutherford Gold Foil Experiment Apparatus
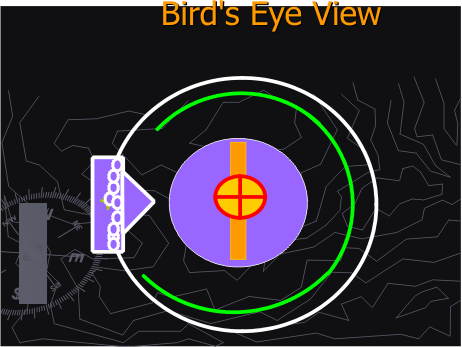
Figure 1B. Rutherford Gold Foil Experiment Apparatus in a cloud chamber
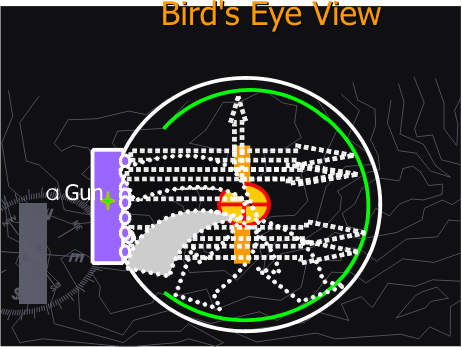
Figure 1C. Alpha particles interacting with a gold atom in the gold foil
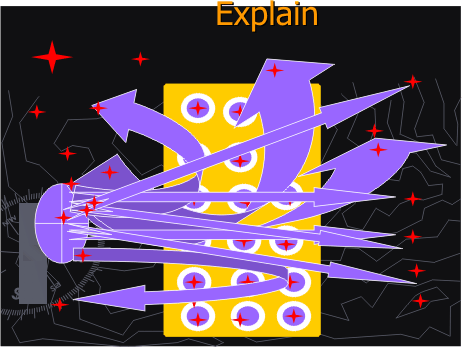
Figure 1D. Alpha particles interacting with gold atoms in the gold foil
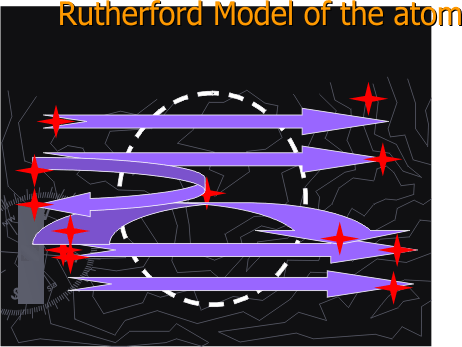
Figure 1E. Bohr Model of the atom
|
1 |
4 |
|
2 |
5 |
|
3 |
6 |
Figure 2 A. Path angles of a-particles
The red patterned ellipse represents the location of the nucleus.
|
7 |
9 |
|
8 |
10 |
Figure 2 B. Path angles of a-particles
The red patterned ellipse represents the location of the nucleus.
|
1 |
4 |
2 |
5 |
|
3 |
6 |
Figure 3 A. Impact patterns of a-particles on photographic plate (xN)
The red patterned ellipse and star represents the location of the nucleus.
Students were required to distinguish between the path angles of a-particles which were 180° and those which were greater than or less than 180°, based on the spot pattern.
|
7 |
9 |
|
8 |
10 |
Figure 3 B. Impact patterns of a-particles on photographic plate (xN)
The red patterned ellipse and star represents the location of the nucleus.
Students were required to distinguish between the path angles of a-particles which were 180° and those which were greater than or less than 180°, based on the spot pattern.