Summer Research Program for Science Teachers
Ben Stevens
Manhattan Center for Science and Math
August 2003
Radioactive Decay and Half-Life
Purpose: Model the rate of decay of radioactive isotopes using a penny model.
Material (per group): Tupperware container (with top)*, 100 pennies, plastic cup, graph paper (one per student), rulers, handout (attached, one for each student), Periodic Table, tables of isotopic decay types and half-lives
*I have found it works well to identify the isotope with a post-it on the top of each groupís Tupperware container. Common isotopes to use are carbon-14, iodine-131, cobalt-60, hydrogen-3, strontium-90, and uranium-238, though any radioactive isotope with a known decay type and half-life can be used.
National Standards Addressed: Content Standard A (science as inquiry), Content Standard B (structure and properties of atoms), Unifying Concepts (use of models)
Objectives: The students will be able to:
1) Describe how the mass of a radioactive isotope changes with time.
2) Describe the factors that affect the rate of radioactive decay.
3) Write nuclear decay equations to represent natural transmutation.
Prior Knowledge: Previous instruction needs to be given in the types of radioactive decay and in the definition of half-life.
Time: 45-60 minutes
Classroom Set-up: Students need to be in groups of 2-4.
Procedure:
1) Each group will perform the activity described on the attached handout. Pennies represent atoms of the given isotope. Any penny that is tails upon flipping has decayed to a new element.
2) Each student will complete the data table, graph, and worksheet questions.
Pre-activity Questions:
1) How does this penny accurately represent radioactive decay?
2) What is inaccurate about the penny model?
During Activity Questions:
1) Are you able which atoms will undergo radioactive decay?
2) Does every atom decay in the same amount of time?
Post-activity Questions:
1) How is each groupís graph similar? How are they different?
2) Does the type of decay particle (alpha or beta) affect half-life?
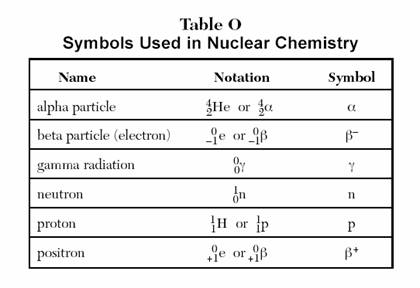
Summary: Review how each radioactive emanation (alpha, beta, positron, gamma) affects the nucleus of an atom.
Extension: Have students investigate the uses and/or dangers of the isotope that they used in the model.
Name ______________________
Radioactive Decay and Half-Life
Instructions: The pennies in your container represent atoms of a radioactive isotope.
1. Seal the container and turn it 4-6 times. This will represent one half-life period.
2. Remove any pennies that come up tails and place then in a cup. These pennies represent those that have undergone radioactive decay.
3. Count the heads up pennies that remain in the original container and record the number in the data table.
4. Repeat steps 1-3 with the remaining pennies for 3 additional half-life periods.
Data:
|
Half-Life Periods |
Time ( ) |
Atoms Remaining |
Mass of Atoms (amu) |
|
0 |
0 |
100 |
|
|
1 |
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
Data Analysis: On the graph paper provided, graph mass vs. time from your data table. Plot all points then connect them with line of best fit. Be sure to label each axis and title your graph.
Conclusions:
1) Write the nuclear decay equation for the radioisotope that you were given.
2) For your isotope, find the amount of time that elapses in 3.5 half-life periods. Show work.
3) Ancient geological formations are often dated by finding the amount of certain uranium isotopes contained in the rock layer.
a. Why are uranium isotopes useful in determining the age of ancient geological formations?
b. How can radioactive dating be useful when the temperatures and pressures that the geological formation has been exposed to have varied so much throughout history?