Summer Research Program for Science Teachers
Partners In Science Program
High School of Economics & Finance
August 2003
This plan relates to the teaching of Avogadro’s number; how the concept came about how the number was determined. In this lab, AP chemistry students will determine Avogadro’s number. This is an excellent lab with which to develop precise measuring skills as well as having students determine one of chemistry’s fundamental parameters.
Before the lab is done, students will form into groups. These groups will be for the entire year. Since these are AP students, I will ask them to design the experiment using the internet as a resource. The students will receive a grade for their experimental proposals.
Once the experimental design is chosen, the equipment will be provided.
Standard A: this will in part be achieved by the lab experience in that students will design own labs (in-school) or engage in real lab give and take
Standard B: this is covered in the textbook
Standard E: Use of lab equipment and how they are used to measure/detect things
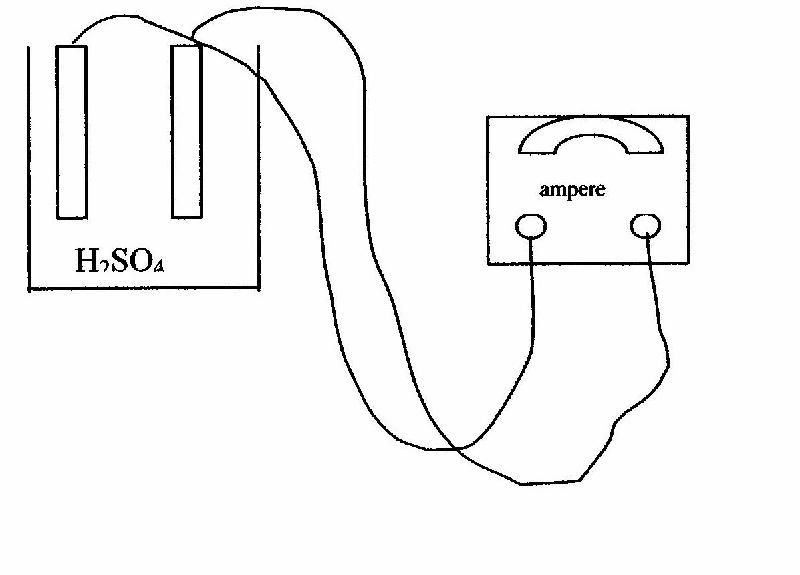
In this experiment, we will use an electrolytic cell.. The major features of this type of cell are:
* An external source of direct current from a battery or power supply.
* Insulated wires to carry the current from the power supply to the electrodes.
* Two electrodes, both of which are made of copper metal.
* A solution of an electrolyte (dilute H2SO4), which will conduct the electric current between the electrodes.
* A diagram of this type of cell is provided:

We will use an ammeter to accurately measure the number of electrons, amperage or current, that has flowed from one of the copper electrodes to the other. We also use a stopwatch to accurately measure the time that this current was flowing. From these two measurements we can determine the number of coulombs that have passed between the two electrodes:
coulombs = amps x seconds
From Millikan's oil-drop experiment, we know the charge on a single electron is 1.60217733 x 10-19 coulombs. So dividing our measured coulombs by the charge on a single electron will give us the number of electrons that flowed between the two electrodes:
number of electrons = coulombs / (1.60217733 x 10-19 coulomb/electron)
Remember that each copper atom actually releases two electrons when it is oxidized, so the number of copper atoms lost from the anode is half the number of electrons just calculated:
number of Cu2+ ions = number of electrons / 2
By carefully weighing the anode before and after electrolysis, we can determine the grams of copper and therefore the moles of copper that were removed from the anode as Cu2+ ions:
moles of Cu atoms = moles of Cu2+ ions = (initial anode weight - final anode weight) / (63.546 g/mol)
Finally, if we divide the number of individual Cu2+ ions by the number of moles of Cu2+, we will have determined Avogadro's number:
1. Identify the anode and cathode in this cell and provide the half-cell reactions that occurred at each one.
2. What was your calculated value of Avogadro’s number and how close was it to the accepted value?
3. Which trial provided you with the most accurate result and why?
4. Why did you need an electrolyte solution? Why not distilled water?
5. What caused the electrolyte to change color?
6. What are the bubbles composed of and how did they get there?