Summer Research Program for Science Teachers
Nelly Zapana
Grade Level: 11th
Topic: Energy in Chemical Reactions
EXOTHERMIC AND ENDOTHERMIC CHANGES
| REACTION EQUATION | NAME OF CHANGE |
EXOTHERMIC OR ENDOTHERMIC |
P.E.
OF REACTANT(S) (MORE/LESS) |
P.E. OF PRODUCT(S) (MORE/LESS) |
P.E. DIAGRAM |
|
| 1 | HgO(S) + heat Hg(1) + 02 (G) | Decomposition | ||||
| 2 | Vaporization | |||||
| 3 | Freezing | |||||
| 4 | Sublimation | |||||
| 5 | Melting | |||||
| 6 | Burning | |||||
| 7 | Crystallization |
Lesson Plan
Aim: What are endothermic and exothermic changes? [9-12 Content Standard B- Chemical reactions]
Objectives:
Materials
Motivation:
Using a bunsen burner, burn a strip of magnesium, and ask students what are the most noticeable products of the reaction? [9-12 Content Standard B- Chemical reactions]
Procedure
1. Develop an equation including the energy product
REACTANTS --------------------> PRODUCTS
Mg(s) + O2 --------------------> 2 MgO + Heat energy
2. Define exothermic reaction
3. Review the conservation law of energy
4. Ask students: From where does the heat energy originate?
Why is the potential energy of the reactants greater than the potential energy of the products?
[9-12 Content Standard B- Conservation of energy]
5. Develop P.E. diagram for the exothermic reaction.
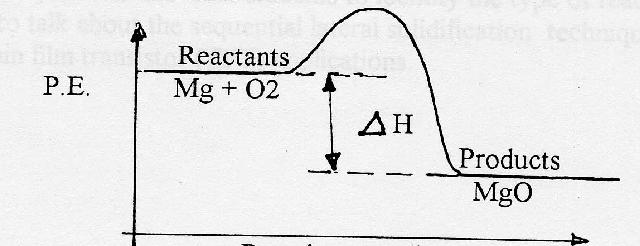
6. Define ![]() ,
,
![]() = PE (Prod) - PE
(React)
= PE (Prod) - PE
(React)
Summary:
Assign numbers to the diagram and ask students to calculate ![]()
Ask the students to complete the following sentence:
For exothermic changes the P.E. of the
product is always ____________ than the P.E. of the reactants and
the sign of ![]() is
always ___________.
is
always ___________.
Demonstration for endothermic reaction
1. Half-fill the test tube with water.
2. Add ¼ test tube of ammonium nitrate to the water and mix.
3. Pass the test tube around the class so the students can feel the coldness. [Content Standard Unifying Concepts- Change, constancy, and measurement]
Procedure:
1. Develop equation on board with energy reactant.
NH4NO3 (s) + H2O (1) + Heat energy ------------------> NH4 N03 (aq)
2. Ask students how the P.E. of the product compares to the P.E. of the reactants in this change?
3. Define endothermic reaction.
4. Develop P.E. diagram for endothermic change.
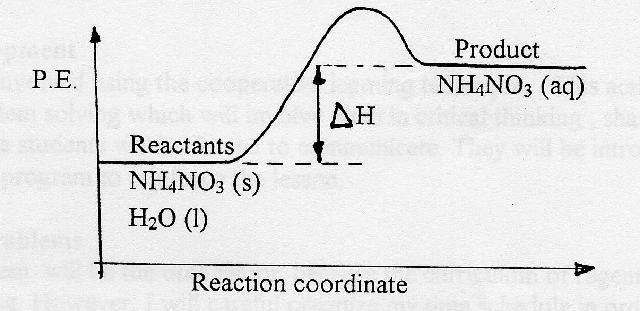
Summary:
As the students to complete the following sentence:
For endothermic changes, the P.E. of the
products is always_______________ than the P.E. of the
reactants and the sign of ![]() is always __________________.
is always __________________.
Explain to the students that explosive crystallization is a mode of crystallization of Si (semiconductor). During irradiation (UV) made by an excimer laser a small region of Si film is heated and melted. This molten layer is cooled relative to the microcrystals with which it is in contact, and crystallizes rapidly into a very fine grained microstructure. The released heat of crystallization causes deeper melting followed by rapid crystallization. Ask the students to identify the type of reaction. This will be a good time to talk about the sequential lateral solidification technique that produces large-grained Si based thin film transistor (TFT) applications.
Return to Chemistry Lesson Plans Menu