BioFuels: The Chemistry and Economics of Alternative Fuels
Garden City High School, Nassau County
Summer Research Program for Science Teachers
August 2008
Course: AP Chemistry, Honors Chemistry, Science Research
Fundamental Goal: Create a single, full year, chemistry project by linking several topics in the curriculum which will be completed at various times during the school year.
Objectives:
Students will learn, understand, and appreciate the following concepts:
Ø Separate matter in different phases.
Ø Determine the density of a liquid.
Ø Calculate the heat of combustion of a liquid using a calorimeter(Thermodynamics)
Ø A real chemical reaction
Ø Reaction Stoichiometry
Ø Identify and draw single, double, and triple covalent bonds.
Ø Learn the process of bonding.
Ø Inter-molecular forces of attraction
Ø Organic Chemistry Synthesis
Ø Environmental Chemistry
Ø Chemistry as related to the Economy
Ø Kinetics and catalysis
Materials, Apparatus, Chemicals:
Separatory Funnels
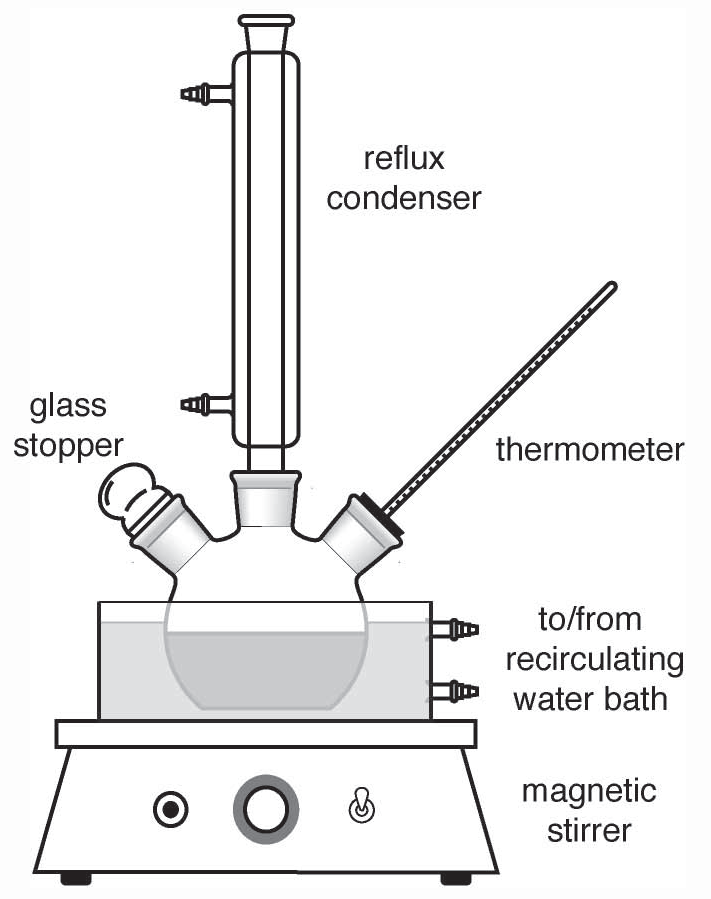
Round Bottom flasks and condensers (See Figure 1.)
TLC Plates
Thermometers
Metal Calorimeters (Coffee and soda cans)
Methanol, ethanol, vegetable oil, motor oil
NaCl
NaOH
Peanut oil( any vegetable oil will work fine also)
Silver Nitrate
Laboratory Notebook
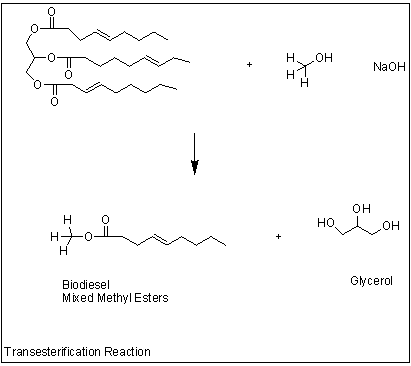
Biodiesel (This will be synthesized)
Procedure:
1)
Making the Biodiesel: Lesson #1
(This Section is intended For Research or
AP CHEMISTRY STUDENTS)
a)
Have several students work in groups of
three and set up the following apparatus. (an alternate suggestion is to have
the teacher do this part)

Figure 1
b)
Based on the chemical reaction in Figure
2, have students determine the Stoichiometry for this reaction. (8:1 mole ratio
of peanut oil to methanol)
c)
Add enough NaOH (catalyst) so that it is
about 1% of the mass of the peanut oil used.
d)
Stir the mixture for about one hour at 600C.
Remove the glycerol from the bottom of the flask with a pipet.
e)
The mixture is allowed to react for
another 30 minutes.
f)
The product mixture is then transferred
to a separatory funnel and washed with water until the pH is neutral. This
ensures that the catalyst is removed from the reaction.
g)
Allow the reaction mixture to stand for 5
days.
h)
The reaction mixture is rewashed with
NaCl(aq) saturated.
i)
Wash with AgNO3(aq)
j)
Remove the biodiesel layer from the
aqueous layer.
k)
Determine its density.
2)
Thin Layer Chromatography: Lesson #2
b)
Determine the Rf values of any
spots and record their value.
c) The eluting solvent can be a 2:1 mixture of hexane ethyl acetate or you can have students determine this.
3)
Heat of Combustion:
Lesson #3
a)
Using a bomb calorimeter, If you are so
blessed by the people who love to give grant money determine the heat of
combustion of the biodiesel.
(The calorimeter shown above might be similar to one that you use to measure the Joule content in a nut or other food.)
The only difference is to replace the tip shown under the calorimeter with some cotton rope.
a)
Determine the mass of the rope
b)
Soak the tip of the rope in the biodiesel
and record this mass.
d)
Pour exactly 100.00ml of water into the smaller soda can and record the initial
temperature of the water.
e)
Ignite the biodiesel as shown above.
f)
Continuously stir the water with the
thermometer until the biodiesel is burned off the rope.
g)
Record the mass of the rope.
h)
Record the final temperatures of the
water.
i)
Repeat a-h for each of the remaining
fuels.
j)
Determine the heat of combustion in
Joules/gram of fuel.
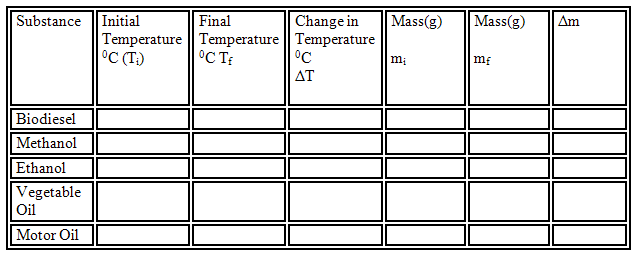
Calculations
Calculate the heat of combustion by using the principle of energy conservation.
Heat Lost by the Fuel= Heat Gained by the
Aluminum can and the Water
mcΔTfuel
= mcΔTH20
+ mcΔTAl
The individual lessons from this project can be as long term and at any level the instructor desires. The questions below can be used or revised to fit you r particular group of students.
Analysis Questions
2) How and why did you determine the density of the biodiesel
3) What do the Rf values from the TLC plates tell you?
4) Determine the Entropy and ΔG values for this reaction.
5) Using standard bond energies determine the heat of combustion of the biodiesel. How does this compare with your experimental value?
6) In terms of bonds breaking and making, how is energy released in this reaction?
7) Which fuel burned the cleanest? Why?
8) How will biofuels affect the big picture of our Environment?
9) Which fuel is the most economical?
10) How will the use of each of these fuels affect our economy? Base your answer on information you have found in reputable journals.
11) Why is the biodiesel washed in AgNO3?
12) From known heats of combustion determine a % error
13) If your value is high or low be concise and explain Why.
Bomb Calorimeter (If Dreams Come True)
Best Method for Measuring Combustion

National Science Standards
Ø Making clear and concise conclusions on the data they accumulate.
Ø Determination if they have taken enough data to draw conclusions about the environment and about the economic plausibility.
Physical Science Standard
Ø Precipitation Reactions
Ø Organic Chemistry
Ø Inter- molecular forces
Ø Bonding
Ø Thermodynamics
Ø Chemical Reactions
Ø Separation of matter
Ø Use of computers to store data, and calculate necessary values.
Ø Use of computer interfaced temperature probes to take temperatures of combustion.