
Rodolfo A. Santos Return to Chemistry Menu
High School of International Business and Finance
Summer 2001
How
can we measure the rate of a chemical reaction?
Primary Objective – Students will realize that chemical reactions can produce physical changes that may be used to quantify the extent of a reaction within an elapsed time, the basis of any kinetic experiment.
Secondary Objective – Students will realize that alcohol molecules affect the rate of the reaction by blocking the effective alka-seltzer and water molecular collisions.)
Method:
Inquiry-based, groups of 4
Materials per group:
3 125 ml Erlenmeyer flasks
3 balloons
3 ties (to seal balloons)
alka seltzer tablets (3)
water
isopropyl alcohol (rubbing alcohol >90%)
graduated cylinder
timer
balance
Query 1. What happens when alka-seltzer is dropped into water?
2. Can this phenomenon be used to determine if a reaction occurred? Why?
3. What happens when alka-seltzer is dropped into almost pure alcohol? [test]
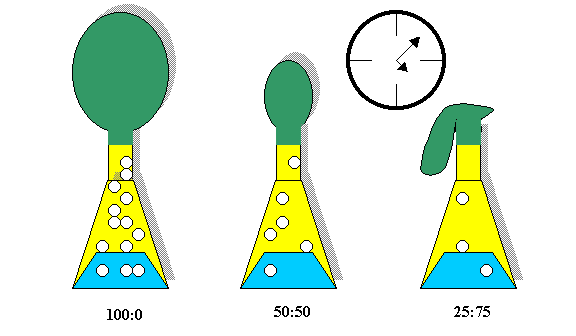
Develop: Allow the groups to design an apparatus using the listed materials that can test the quantity of carbon dioxide produced after a fixed amount of time from 3 differing concentrations of an alcohol/water mixture. The concentrations of water to alcohol are 100:0, 50:50, 25:75. [Written on the board]
The working apparatus and experiment should look similar to the figure below.


Groups will recognize that the amount of gas produced is proportional to the amount of alka-seltzer consumed. The gas liberated has weight (and volume!). This physical property can be measured by tying the balloons and weighing them (a careful experimenter would recognize the need for weighing the empty balloons first and subtracting this value from the balloon plus carbon dioxide.)
At this stage the experiment is done.
Calculation:
raten
= Weightn/(elapsed time)
Compare results (discussion).
Final Question:
Why does alcohol affect the rate of the alka-seltzer/water reaction?
(Answer: Collision Theory)