 |
Using Flame Tests to Identify Unknowns
New Rochelle
High School, W
Summer Research Program for Science Teachers
August 2011
Subject:
C
Grade Level: 9 - 12
Unit: Atomic Theory & Structure
Time Required: One 48 minute period plus time at home for analysis questions to be completed
Purpose: Students will investigate how the electromagnetic spectrum can be used to identify the unknown composition of a Colorflame candle by performing flame tests.
Objectives: SWBAT:
Explain how the electromagnetic spectrum is produced
Use observation to compare and contrast different spectra produced
Identify the element(s) present in a Colorflame candle
Apply knowledge of electron energy and the electromagnetic spectrum to the chemistry of fireworks.
Aim:
To
identify the metal in a Colorflame candle by comparison to known compounds
Materials:
Colorflame Candles
5 known samples of metal-containing compounds
Wooden splints
Bunsen burner
Matches
Beaker of cold water
Colored Pencils
Video: How It's Made – Fireworks ( link here)
Materials note:
When choosing the 5 known metal samples, consult MSDS for flammability or
inhalation hazards!
Introduction:
Energy can be added to atoms many
different ways. It can be in the form of light, an electric discharge or heat.
This added or extra energy is emitted when the excited electrons in the atoms
give off light and fall back to lower shells. The light emitted has wavelengths
and colors that depend on the amount of energy originally absorbed by the atoms.
Usually each individual excited atom will emit one type of light. Since we have
billions and billions of atoms we get billions of excitations and emissions
The energy
levels in atoms and ions are the key to the production and detection of light.
Energy levels or "shells" exist for electrons in atoms and molecules. The colors
of dyes and other compounds results from electron jumps between these shells or
levels. The colors of fireworks result from jumps of electrons from one shell to
another. Observations of light emitted by the elements is also evidence for the
existence of shells, sub shells and energy levels.
Different elements emit different emission spectra when they are excited because
each type of element has a unique energy shell or energy level system. Each
element has a different set of emission colors because they have different
energy level spacings. We will make qualitative observations of the emission
spectra, or pattern of wavelengths (atomic spectra), emitted by five different
elements in this lab. While we will not be looking the full spectra produced,
the color that we can see with our naked eye can help us to identify the
wavelength of light produced. We will then identify an unknown element by
comparing the color of the unknown with the flame color of our known samples. If
you miss anything, additional information and a virtual flame test can be found
here:
http://www.800mainstreet.com/spect/emission-flame-exp.html
What metals do colors indicate?
|
Color |
Metal |
|
Red |
Carmine:
Lithium compounds. Masked by barium or sodium. |
|
Yellow |
Sodium compounds,
even in trace amounts. A yellow flame is not indicative of sodium unless
it persists and is not intensified by addition of 1% NaCl to the dry
compound. |
|
White |
White-Green:
Zinc |
|
Green |
Emerald:
Copper compounds, other than halides. Thallium. |
|
Blue |
Azure:
Lead, selenium, bismuth, CuCl2 and
other copper compounds moistened with hydrochloric acid. |
|
Violet |
Potassium
compounds other than borates, phosphates, and silicates. Masked by
sodium or lithium. |
Procedure
1.
Light your Colorflame candle.
Record the color you observe.
2.
Blow out your candle.
3.
Light the bunsen burner
4.
Take a small amount of known
sample on a clean wooden splint.
5.
Wave the splint through the
flame. DO NOT HOLD IT IN THE FLAME.
6.
Record the color you see.
7.
Dip the splint in cold water to
extinguish.
8.
Repeat with other known
compounds, using a clean splint each time.
9.
Turn off gas to burner and clean
up lab area.
Data
Analysis:
1.
Identify the metal your candle
contained. Explain how you know.
2.
a) Describe, in terms of
subatomic particles, how the light you observed in produced.
b) What region of
the electromagnetic spectrum is this light found? Identify the specific
wavelength range that this light
could be found at.
c)
Using the visible light spectrum, estimate the wavelength and frequency of your
unknown.
d)
Using your estimates in c, calculate the energy produced by the light.
3.
Arrange the following types of
light in order of energy: x-rays, vis light, gamma rays, radio waves, UV
4.
From the August 2008 Regents
exam.
Base your answers to questions 66 through 68 on the information below.
In a laboratory, a glass tube is filled with
hydrogen gas at a very low pressure. When a scientist applies a high voltage
between metal electrodes in the tube, light is emitted. The scientist analyzes
the light with a spectroscope and observes four distinct spectral lines. The
table below gives the color, frequency, and energy for each of the four spectral
lines. The unit for frequency is hertz, Hz.
Visible Spectrum of Hydrogen
Color
Frequency
(x 1014 Hz)
Energy(x
10–19 J)
red
4.6
3.0
blue green
6.2
4.1
blue
6.9
4.6
violet
7.3
4.8
66. On a separate sheet of graph paper or electronically, plot the data from the
data table for frequency and energy. Circle and connect the points [1]
67. A spectral line in the infrared region of the spectrum of hydrogen has a
frequency of 2.3 x1014
hertz. Using your graph, estimate the energy associated with this spectral line.
[1]
68. Explain, in terms of subatomic particles and energy states, why light is
emitted by the hydrogen gas. [1]
5.
From the June 2011 Regents exam. Base your answers to questions 52 through 54 on
the information below:
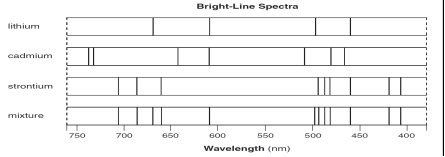
The bright-line spectra for three elements and a mixture of elements are shown
below.

52. Explain, in terms of both
electrons and energy, how the bright-line spectrum of an element is produced.
[1]
53. Identify all the elements in the mixture.
[1]
54. State the total number of valence electrons in a cadmium atom in the ground
state. [1]
6.
Suppose you were a firefighter and you were called to a chemical plant fire.
Upon arrival you see a bright violet/purple flame. What chemical would that tell
you is burning?
7.
After viewing the video on fireworks, use the information in your data table to
design a firework and then predict what it would look like as it burns. Within
the firework, specify which metal(s) your firework contains. Along the fuse,
draw the fire as it burns.
Assessment:
Students will be assessed on participation in
experimentation and final lab write up. An exit card will be given with the
following question to be completed before leaving the lab: In one sentence -
describe, in terms of energy and electrons, how a firework functions.
National Science Education Standards Grades 9 to 12
NS.9-12.1 Science As Inquiry
As a result of activities in grades 9-12, all students
should develop abilities necessary to do scientific inquiry and understandings
about scientific inquiry
NS.9-12.2 Physical
Science
As a result of their activities in grades 9-12, all
students should develop an understanding of
Standard 1: Students will use mathematical analysis, scientific inquiry, and engineering design, as appropriate, to pose questions, seek answers, and develop solutions.
Mathematical Analysis Key Idea 1: Abstraction
and symbolic representation are used to communicate mathematically
Scientific Inquiry Key Idea 1: The central
purpose of scientific inquiry is to develop explanations of natural phenomena in
a continuing, creative process.
Key Idea 3:
The observations made while
testing proposed explanations, when analyzed using conventional and invented
methods, provide new insights into phenomena.
Standard 4:
Students will understand and apply scientific concepts, principles, and
theories pertaining to the physical setting and living environment and recognize
the historical development of ideas in science.
Standard 7:
Students will apply the knowledge and thinking skills of mathematics, science,
and technology to address real-life problems and make informed decisions.